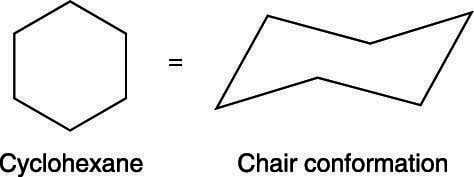
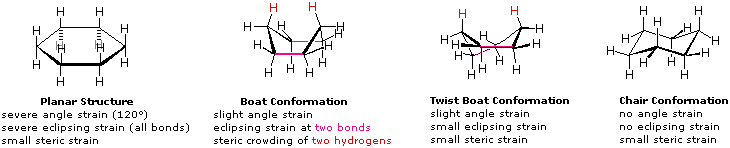
Cyclo hexane is more stable in chair form than in boat form due to following reasons in chair form c-h bonds at adjacent carbons are staggered but in boat form some c - h bonds are fully eclipsed in boat form there is repulsion between flagpole hy.... This organic chemistry video tutorial focuses on the chair conformation of cyclohexane. it shows how to draw the most stable conformation. it contains a few notes, examples, and practice problems. We begin by studying the most stable conformation of cyclohexane, which has completely staggered dihedral angles at each of the six c-c bonds. this conformation is not flat but is folded into the shape of a lawn chair, so it is called the chair conformation..
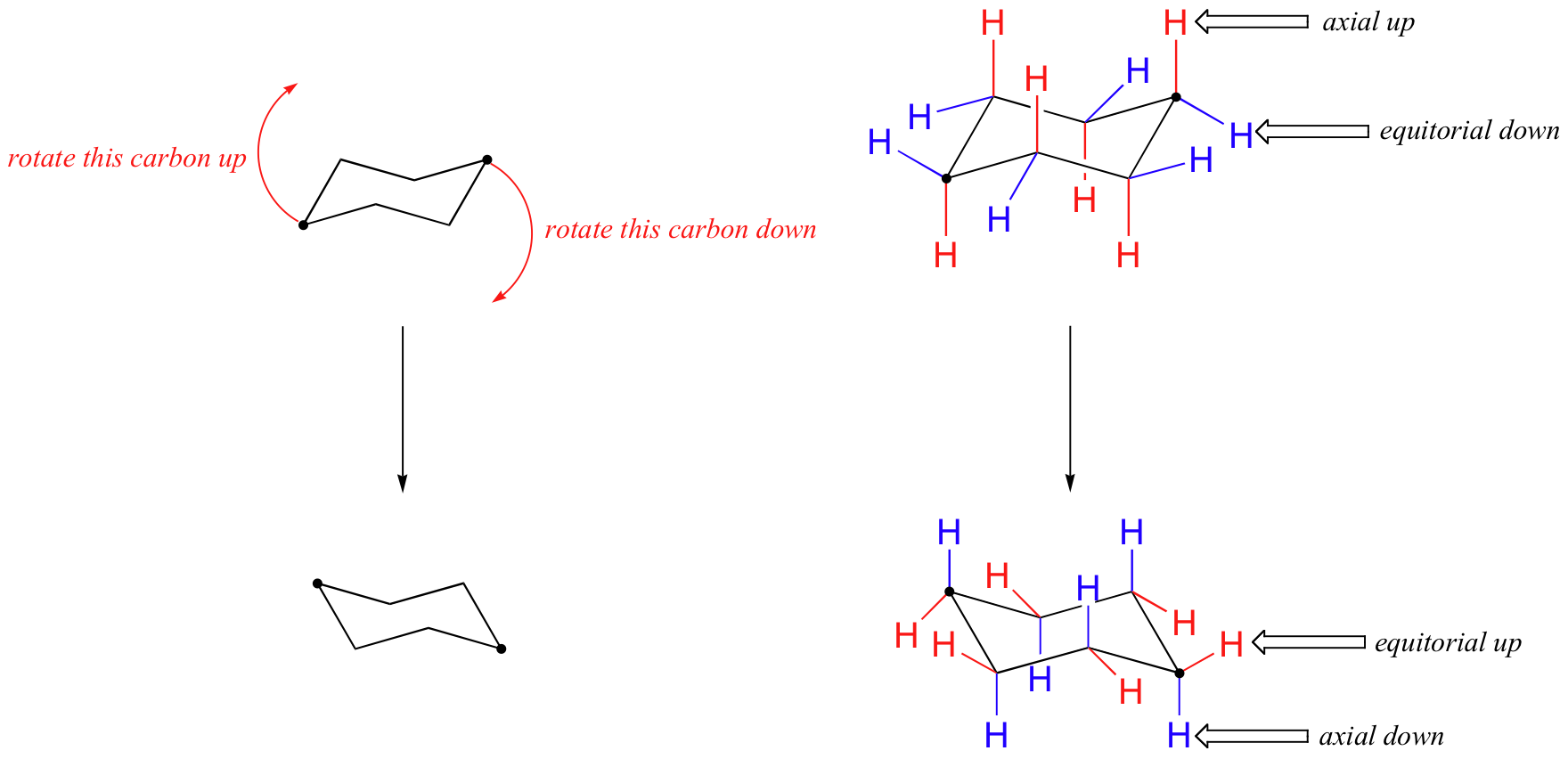
The chair conformation is a lot more stable. if you have cyclohexane in the boat form the tips of the boat will be close to one another, and they would repulse.. Conformation. in the pictures below, the methyl in the equatorial position is more stable because it avoids interaction with the hydrogen atoms. the larger the group is, the more it will tend to remain in the equatorial position. therefore, when trying to determine which chair is more stable, place the larger group in the equatorial position.. Chair and boat shapes for cyclohexane. well, what's more stable? that's actually one of the main points of being able to visually think about the three dimensional structure of any of these hydrocarbons, or in this case cyclohexane. from this ch2 as possible. so in that situation, we have a lower potential energy, or it is a more stable.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.